If you love doing experiments in the laboratory, you probably have heard about pure water.
Is pure water an element, compound, or mixture?
Pure water that contains only H2O molecule is a compound. It is composed of two different elements i.e. hydrogen and oxygen that bond chemically in a fixed proportion. Thus, pure water is considered a compound.
Pure water is different with the common water.
Pure water can be produced by boiling the water and then cooling back the steam. This process is conducted to eliminate the minerals and salt that is naturally contained in regular water.
This article will talk about why pure water is considered a compound as opposed to an element or mixture.
So, keep on reading!
What Is An Element?
Element in science is a simple matter that only has its own of atom without any addition. It purely consists of a single type of atom so it cannot be broken down.
Some examples of elements are lead, tin, carbon, and oxygen.
An element can combine with other elements to produce other form of matter i.e. compound or mixture.
For example, pure iron is an element, carbon is also an element. When both of them combine, they make steel, which is a mixture.
What Is A Compound?
Compound is another type of pure substance, but it contains more than one element. Those elements bond chemically in a certain fixed proportion.
Compound happens when two or more elements combine and form chemical bonds. A compound can be broken down into simpler form but you need chemical reaction do it.
Compound has fixed ratio of composition. It means that the same kind of compound will always have the same kind of molecule no matter where it’s found on earth.
For example, table salt (NaCl) is a compound that consists of Sodium and Chlorine. The ratio of salt molecule is always 1:1.
What Is A Mixture?
When two or more elements or compounds combine physically in no fixed proportion, they make a mixture.
A mixture is a matter that happens when two or more substances mixes in without definite ratio of composition.
An example of a mixture is brass. Brass is made by combining copper (Cu) and zinc (Zn), sometimes other elements are also mixed in. The proportion of copper, zinc, and other elements in brass is not exactly the same from one brass to another. That’s why brass comes in various kinds.
Is Water A Compound or Mixture?
Regular water is a mixture because it consists of various substances that combine physically in no fixed proportion.
Water that is naturally found in our daily life is considered a mixture because it consists of different substances that combine physically. It contains the H2O molecule itself but it also contains other things. It might have minerals such as calcium, sodium, iron, etc.
The true H2O (purest form of water) isn’t available naturally on earth. You need to extract it from the regular water.
Scientifically, pure water is not possible. It’s difficult to have the purest form of H2O on earth because water love dissolving something in it.
Is Pure Water A Compound or Mixture?
The true pure water (H2O) is a compound because the constituents are bond chemically in a certain fixed proportion. H2O is not a mixture because a mixture doesn’t have a definite ratio of composition.
‘Pure water’ is actually a tricky term.
What the industry consider as pure water is generally a water that has experienced mechanical process to remove the impurities. It may also mean a drinking water that is free or has reduced amount of bacteria in it.
In that case, pure water might still contain little amount of trace materials in it. We might not see it, but there is a high chance that something is there. It might be trace minerals or other substances. If that’s the case, then pure water is a mixture because it has no definite ratio of composition.
However, the scientific version of pure H2O might be different. If we are talking about the purest form of water i.e. the H2O molecule itself, then it’s classified as a compound.
H2O as A Chemical Compound
H2O is a chemical compound that is composed of hydrogen and oxygen. It’s colorless and odorless.
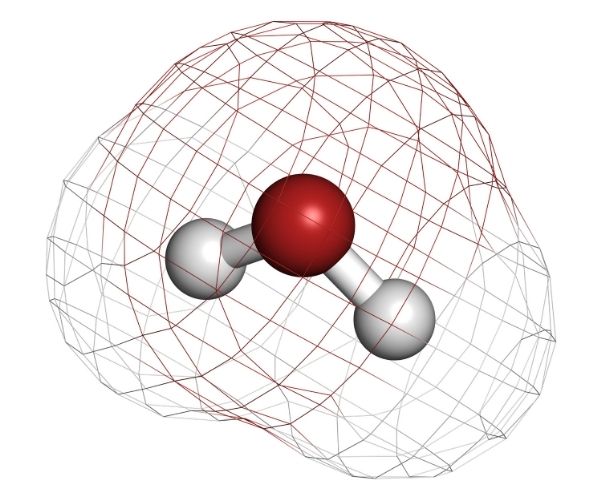
The molecule of H2O has 2 hydrogen atoms and 1 oxygen atom that form 2 covalent bonds. The proportion of the constituent is fixed, with the ratio of 2:1.
Some of the chemical properties of H2O are:
| Chemical formula | H2O |
| Molar mass | 18.01528 g/mol |
| Boiling point | 100 degree Celcius |
| Melting point | 0 degree Celcius |
| pH | 7 (neutral) |
| Density | 997 kg/m3 |

![Is Baking Soda An Element, Compound, or Mixture? [ANSWERED]](https://dearlearners.com/wp-content/uploads/2021/01/is-baking-soda-an-element-compound-or-mixture_-768x480.jpg)
![Is Coffee an Element, Compound, or Mixture? [ANSWERED]](https://dearlearners.com/wp-content/uploads/2021/02/is-coffee-an-element-compound-or-mixture_-768x480.jpg)
![Is Ink An Element, Compound, or Mixture? [ANSWERED]](https://dearlearners.com/wp-content/uploads/2021/02/is-ink-an-element-compound-or-mixture_-768x480.jpg)
![Is Vinegar An Element, Compound, or Mixture? [ANSWERED]](https://dearlearners.com/wp-content/uploads/2021/01/is-vinegar-an-element-compound-or-mixture_-768x480.jpg)
![Is Brass An Element, Compound, or Mixture? [ANSWERED]](https://dearlearners.com/wp-content/uploads/2021/01/is-brass-an-element-compound-or-mixture_-768x480.jpg)